Heavy! Weishan Bio-intestinal cancer fecal DNA early detection product Changbeishan obtained EU CE certification
- Classification:Technical Column
- Author:
- Source:
- Release time:2021-07-09
- Visits:0
[Summary]
Heavy! Weishan Bio-intestinal cancer fecal DNA early detection product Changbeishan obtained EU CE certification
[Summary]
- Classification:Technical Column
- Author:
- Source:
- Release time:2021-07-09
- Visits:0
Recently, Changbeishan®, a colorectal cancer stool DNA early detection product independently developed by VersaBio, successfully completed the EU CE filing and obtained a license for EU market circulation.

Changbeishan has a sensitivity of 67% for advanced adenomas, a sensitivity of 93% for early colorectal cancer (stage I-II), and a detection specificity of 92%. The approval of the EU CE filing means that Weishan Biotech has gained wider market recognition, accelerated the company’s internationalization process, and distanced the company’s vision of becoming the world’s leading precision medicine/early disease screening supplier. All Chinese people are one step closer to getting accurate, fast, and affordable health services.
Since its establishment, the company has been focusing on the R&D and industrialization of non-invasive early screening of gastrointestinal tumors. It is the first company in the industry to have both blood and stool DNA methylated colorectal cancer screening products, and has the international leading "complex Core technologies such as sample pretreatment, “methylation speed detection”, “accurate nucleic acid capture and amplification”, and “methylation targeting enhancement”. At present, 33 patents have been applied for (10 authorized), 1 PCT patent, 12 SCI papers published, 5 soft works, and related technologies won the first prize of IVD group of the 3rd China Medical Device Innovation and Entrepreneurship Competition finals and a series of awards. . The company will take the EU CE certification as a new starting point, continue to overcome difficulties, and strive to achieve more significant results in the field of early colorectal cancer screening, and make due contributions to human health services.
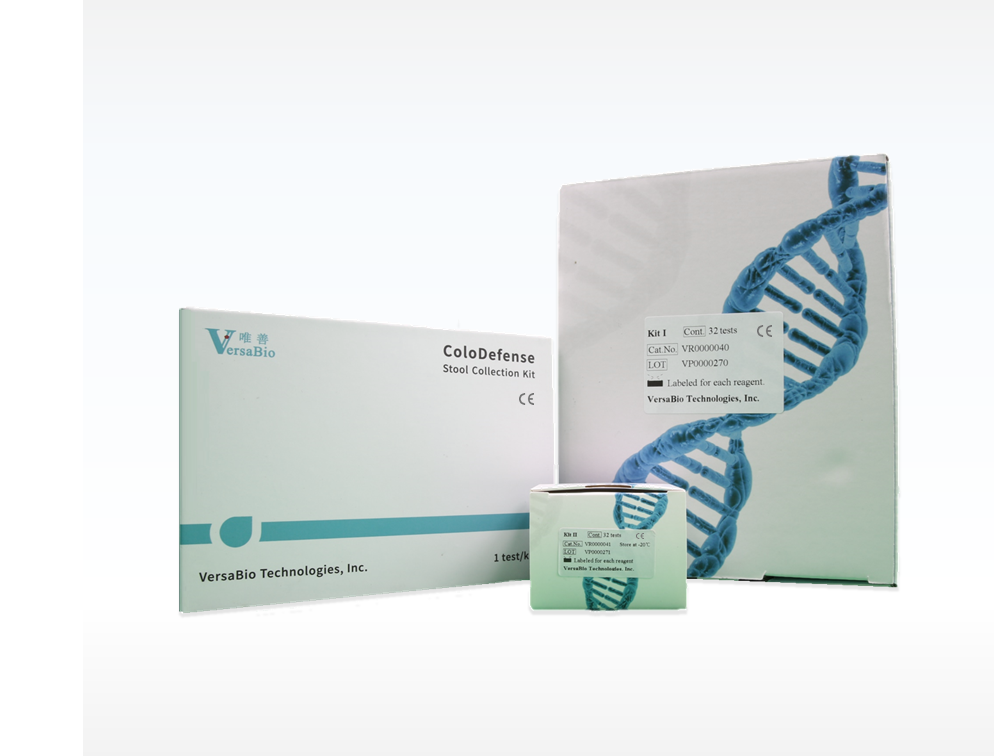
• Two products that have obtained notification certificates issued by authorized representatives of the European Union
ColoDefense Stool Test (Kit I, II)
Stool Collection Kit
掃二維碼用手機看
CONTACT US
Tel:0512-36680291
17751718557
E-mail:versabioreport@sina.com
Add:Room 207, Building 1, No.232 Yuanfeng Road, Kunshan City, Jiangsu Province